Press Release: Avilex Pharma and Simcere announce strategic partnership
Press Release: Avilex Pharma and Simcere announce strategic partnership
Avilex Pharma and Simcere announce strategic partnership to develop and commercialize novel treatments for acute ischemic stroke in greater china
Copenhagen, Denmark, December 17, 2021
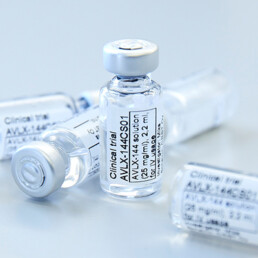
Avilex Pharma ApS (Avilex), a clinical-stage biotechnology company developing innovative medicines for neurological disorders, and Simcere Pharmaceutical Group Ltd (HKEX: 2096) (Simcere) today announced that they have entered into a strategic partnership to develop and commercialize Avilex’ lead program AVLX144 in Greater China.
The agreement grants Simcere an exclusive regional license to develop and commercialize AVLX-144, Avilex’ Phase 2-ready dimeric PSD-95 inhibitor, for the treatment of acute ischemic stroke (AIS) and other neurological conditions, as well as access to certain of the company’s library of early peptide candidates. Under the terms of the agreement, Avilex Pharma will receive an undisclosed upfront payment and will also be eligible for payments upon achievement of certain development and sales milestones, with all components amounting to a total of over USD 175 million (DKK 1.15 billion). In addition, Avilex Pharma will receive royalties on sales. Further financial details were not disclosed.
“We are extremely pleased to be entering into this partnership with Simcere as they are one of China’s leading pharmaceutical companies, and have established exceptional capabilities in developing and launching effective therapies for AIS. We have full confidence in the Simcere team in bringing our life-saving medicines to patients in need,” says Kristian Strømgaard, Ph.D., Chief Executive Officer at Avilex Pharma.
“As a founding investor of Avilex we are extremely pleased with this important partnership with Simcere which will expedite the development of AVLX-144 in China to the benefit of patients suffering from AIS. Furthermore, this collaboration validates Avilex’ differentiated approach for targeting PSD-95, a clinically validated target for AIS, and will further fuel our internal efforts to develop unique second generation compounds towards clinical development,” says Morten Graugaard Døssing, Partner at Novo Holdings.
About Avilex Pharma
Avilex Pharma is a clinical stage biotech company developing a novel class of neuroprotectants based on inhibiting PSD-95, a clinically validated mechanism in the treatment of AIS. The company’s proprietary dimeric ligands target two protein modules of PSD-95 simultaneously, leading to key advantages, such as exceptionally high affinity to PSD-95, excellent stability, and enhanced neuroprotective properties in vivo. Avilex Pharma is also developing next-generation plasmin-resistant PSD-95 inhibitors advancing rapidly through preclinical development
Avilex is a start-up from the University of Copenhagen, established by Novo Holdings, and supported by funding from the Wellcome Trust, the Danish Innovation Fund, and the Danish Growth Fund. About Simcere Pharmaceutical Group Limited Simcere Pharmaceutical Group Limited is rapidly transitioning to an innovation and R&D-driven pharmaceutical company, with a mission of “providing today’s patients with medicines of the future.” It has established a national key laboratory of translational medicine and innovative pharmaceuticals. Simcere has
a diversified product portfolio in strategically focused therapeutic areas, including oncology, central nervous system diseases and autoimmune diseases, with leading positions in their respective therapeutic segments and/or established track record. Its vigorous in-house R&D efforts and extensive R&D collaborations have made it a strategic cooperation partner with world leading pharmaceutical companies and biotechnology companies, in an effort to bring more global life science breakthroughs to China. For more information,
please visit www.simcere.com.
Kristian Strømgaard, Professor, PhD
Phone: (+45) 5123 6114
Successfully completes phase 1 clinical trial

Successfully completes phase 1 clinical trial
Avilex Pharma successfully completes its phase 1 clinical trial of AVLX-144 – A potentially game-changing treatment for acute neurological conditions
Avilex Pharma, a clinical stage biotechnology company developing innovative treatments for acute neurological conditions such as acute ischemic stroke (AIS) and subarachnoid haemorrhage (SAH), is excited to announce the successful completion of the company’s first-in-human Phase 1 clinical trial of its lead compound AVLX-144.
During the course of the Phase I study, AVLX-144 was well-tolerated and demonstrated no noteworthy safety issues related to treatment The result provides a solid foundation for Avilex’s transition into later phase clinical trials to further study the compound’s efficacy.
“We are extremely pleased with the successful outcome of our first-in-human study, firstly being able to conduct and finalize the Phase 1 study under the challenging Covid19 conditions, and secondly as the results obtained are as good as we could have hoped for.” says Mikael Thomsen, Chief Development Officer at Avilex Pharma
Kristian Strømgaard, Ph.D., Chief Executive Officer at Avilex Pharma adds “This is a major milestone for Avilex. As we think back to all the hard work that went into this achievement and celebrate it, we are also excited about what this means for patients moving forward as we progress through the next phases of clinical development and continue on a path to providing a safe and efficacious treatment option for patients,”
The Phase I single ascending dose study was conducted in collaboration with Clinical Research Services Turku (CRST) in Finland. The study examined the safety, tolerability, and pharmacokinetics of AVLX-144 and identified the recommended Phase 2 dose. Avilex Pharma plans to initiate a Phase 2 trial with the primary objective of evaluating the clinical efficacy of AVLX-144.
About Avilex Pharma
Avilex Pharma is a clinical stage biotech company developing a novel class of neuroprotectants based on inhibiting PSD-95, a clinically validated mechanism (ref), using proprietary dimeric ligands that target two protein modules of PSD-95 simultaneously. This strategy has led to the identification of lead compound AVLX-144, whose specific design provides key advantages, such as exceptionally high affinity to PSD-95, excellent stability, and enhanced neuroprotective properties in vivo.
The AVLX-144 program was supported by funding from the Danish Innovation Fund Grand Solutions Program and the Wellcome Trust Translational Award, as well as investments from Novo Holdings and Vækstfonden.
Kristian Strømgaard, Professor, PhD
Phone: (+45) 5123 6114
Promising Agent for Ischemic Stroke Now to Be Tested in Humans

Promising Agent for Ischemic Stroke Now to Be Tested in Humans
A compound developed by Danish researchers from the University of Copenhagen could be the key to one of the greatest unsolved challenges in modern medicine. The compound, which has shown very promising effects in animal models of ischemic stroke, is now to be tested in humans and also serves as a template for development of a marker for ischemic stroke.
Ischemic stroke is one of the most frequent causes of death in the Western world and the primary cause of long-term disability such as paralysis and loss of function. In addition, ischemic stroke is one of the costliest diseases for Western countries often involving comprehensive hospital treatment, expensive medicine, rehabilitation, and long-term sick leave.
To date, only one drug, Alteplase, has been approved for the treatment of ischemic stroke. Nevertheless, the use of the drug requires a brain scan and acute treatment, which means that less than 10 percent of patients affected by ischemic stroke are actually treated.
Thus, treatment of ischemic stroke is one of the greatest challenges in modern medicine today.
New compound can be a paradigm shift in the treatment of ischemic stroke
A compound denoted AVLX-144, originally discovered at the University of Copenhagen and subsequently developed by the Danish biotech company Avilex Pharma, has recently shown very promising results for the treatment of ischemic stroke.
The compound represents a new concept in the treatment of brain disorders, and preclinical studies have reinforced the expectation that AVLX-144 could become a drug for the acute treatment of ischemic stroke.
In a large, newly established, multiyear, international project supported by Innovation Fund Denmark, a group of experts from Denmark and abroad will collaborate to test AVLX-144 in human Phase 1 clinical trials.
The team will also develop a marker (PET tracer) for investigating whether AVLX-144 binds to the specific protein in the brain for which it was originally designed. Such a marker will be crucial for the further development of AVLX-144 and can also potentially be used for the diagnosis of patients who have been affected by ischemic stroke.
“We consider the investment from Innovation Fund Denmark a huge recognition of our previous work on developing AVLX-144. The investment will bring us a very decisive step forward in development, and we are convinced that if these studies turn out as we hope, it will result in great interest from investors and pharmaceutical companies. Ultimately, we can bring a paradigm shift in this type of treatment and help the millions of people affected by ischemic stroke each year.” says Kristian Strømgaard, co-founder of Avilex Pharma and Professor at the University of Copenhagen.
Facts
Program: Grand Solutions
Innovation Fund Denmark’s investment: DKK 20 million
Total budget: DKK 26 million
Duration: 2.5 years
Official title: AVLX-144 as a novel treatment for ischemic stroke
About the project
The project is a collaboration between Danish biotech company Avilex Pharma, the University of Copenhagen, Rigshospitalet, the University of Southern Denmark and Finnish Turku University. Together, the parties will examine AVLX-144 in clinical trials and develop a marker for diagnosis of blood clots in the brain based on the drug compound.
Press release translated from following source: https://innovationsfonden.dk/da/nyheder-presse-og-job/lovende-middel-mod-blodpropper-i-hjernen-skal-nu-testes-pa-mennesker